By Dave Hadfield, VP Customer Solutions with editorial support from Jon Nelson, VP Operations
Dave Hadfield
Recent Posts
Medical Devices with Super Hero-like powers?
Tags: Medical Devices, Future of Medical Devices, Medical Devices with super powers, Life Sciences
Just how resilient is your Medical Device?
I attended COFES 2014 this past week. It was an excellent event bringing together some of the world’s best engineering minds.
Tags: Medical Device Manufacturing, Medical Devices, Device Manufacturing, Product Development, COFES
Feel like your requirements aren't working for you? This might be why
Dedicated requirements management tools are available to manage product, user, system requirements for new product introduction projects. These tools can be feature rich and very good, at least within the isolated context for which they were intended. For example, with a dedicated requirement management tool it’s possible to define a hierarchical set of requirements starting with a product concept, through critical-to-quality user needs and down to detailed functional requirements. Another key feature is tracking test cases and interfacing those with the tool, often it’s possible to automate the generation of traceability reports and validation protocols, whose accuracy is paramount.
Tags: requirements management, requirements gathering, PLM requirements, requirements integration
How PLM Can Cut Manufacturing Costs
Tags: PLM ROI, manufacturing cost savings, PLM and manufacturing, product lifecycle management
Addressing the Growing Complexity of Medical Device Regulatory Requirements
Global regulatory is becoming a very strategic problem for medical device companies. The landscape has become more complex as each agency has matured in their requirements. These continuously evolve and the change to them and change to products must be considered continuously to determine if re-filing is required.
This makes the difference between being able to sell hips, catheters and MRI machines in China, EU, Brazil, Argentina, etc. A PLM centric approach can enable companies to accelerate registration in those regions. This equates to massive revenue opportunities.
This is enabled by having a single copy of the submission data that is accessible in collaborative environment and can be quickly and reliably reused between regions.
Tags: Regulatory Submission, Regulatory Compliance, FDA, global regulatory requirements, international regulatory standards, complex plm processes
PLM contrasts with two traditional approaches to managing the lifecycle of a product, which include:
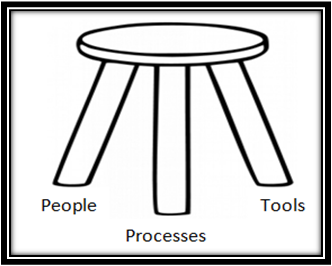
When building your Quality Management System (QMS) for your Medical Device Company, use this simple guide to develop the foundational three-legged-stool. Realize that building an ideal QMS takes more than a good quality manual. We must consider people, processes, and tools as critical components of a system that comes together in harmony.
Tags: Regulatory Compliance, product lifecycle management, Total product lifecycle managment, Quality Management System, QMS