I attended COFES 2014 this past week. It was an excellent event bringing together some of the world’s best engineering minds.
Just how resilient is your Medical Device?
Tags: Medical Device Manufacturing, Medical Devices, Device Manufacturing, Product Development, COFES
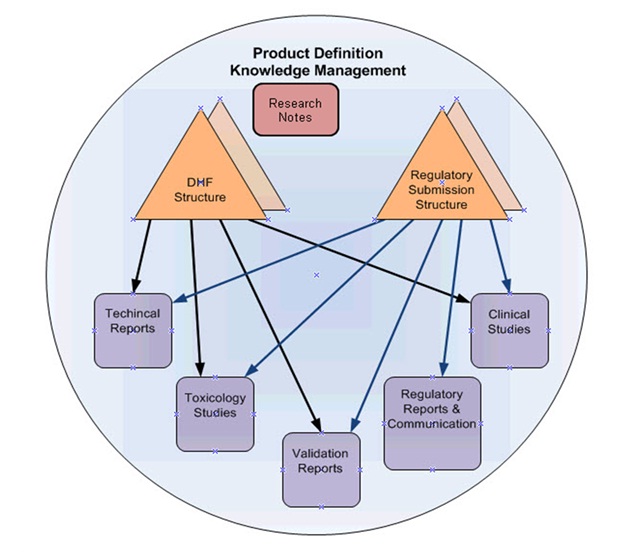
Product Definition Knowledge Management (PDKM) is a solution architecture approach that guides the use of PLM in supporting the full lifecycle of a product. The purpose is to have all the relevant product information in one place, connected to meet business needs, and safe. In other words, PDKM is a single source of the truth. The PDKM solution covers the product definition scientific and engineering information from the earliest research through all the improvements made to the product and its manufacturing process until the end-of-life.
Tags: 510(k), PDKM, NDA, PLM, Product Development, design history file, Product Definition Knowlege Management, plm solution, PLM system, DHF, clinical studies, Product lifecycle, 21 CFR 820.30