Global regulatory is becoming a very strategic problem for medical device companies. The landscape has become more complex as each agency has matured in their requirements. These continuously evolve and the change to them and change to products must be considered continuously to determine if re-filing is required.
This makes the difference between being able to sell hips, catheters and MRI machines in China, EU, Brazil, Argentina, etc. A PLM centric approach can enable companies to accelerate registration in those regions. This equates to massive revenue opportunities.
This is enabled by having a single copy of the submission data that is accessible in collaborative environment and can be quickly and reliably reused between regions.
Addressing the Growing Complexity of Medical Device Regulatory Requirements
Tags: Regulatory Submission, Regulatory Compliance, FDA, global regulatory requirements, international regulatory standards, complex plm processes
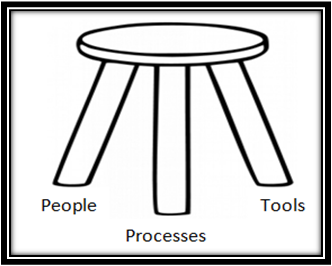
When building your Quality Management System (QMS) for your Medical Device Company, use this simple guide to develop the foundational three-legged-stool. Realize that building an ideal QMS takes more than a good quality manual. We must consider people, processes, and tools as critical components of a system that comes together in harmony.
Tags: Regulatory Compliance, product lifecycle management, Total product lifecycle managment, Quality Management System, QMS
Justifying PLM: A New Perspective on the Investment for Life Sciences Companies
I have been calling directly into the Life Sciences (LS) space for about a year and a half. I will not call myself an expert, but given that I have been in the business world for over 30 years, I feel I have crossed over into the category of a “gray hair” and therefore I can have some relevant observations. My below observations are in some ways provided as an outsider looking into the LS industry.
Tags: Integware, Life Sciences PLM, Regulatory Compliance, PLM Investment, Life Sciences, product lifecycle management, Total product lifecycle managment, Quality Management System, QMS
What do PLM and Tim Tebow have in common?
Tags: NCR, PLM, Integware, Quality system management, Life Sciences PLM, Regulatory Compliance, BOM Management, PLM Insider, Complaint Management, CAPA, Quality Documentation, Life Sciences, PLM implementation, Change Control, product lifecycle management, Total product lifecycle managment, PLM system, PLM deployment, Document Management, PLMinsider, Audit Management, FDA Audits