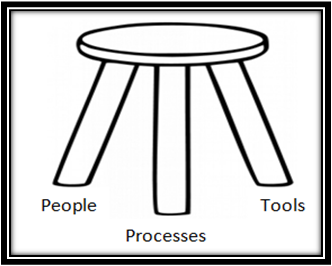
When building your Quality Management System (QMS) for your Medical Device Company, use this simple guide to develop the foundational three-legged-stool. Realize that building an ideal QMS takes more than a good quality manual. We must consider people, processes, and tools as critical components of a system that comes together in harmony.
Tags: Regulatory Compliance, product lifecycle management, Total product lifecycle managment, Quality Management System, QMS
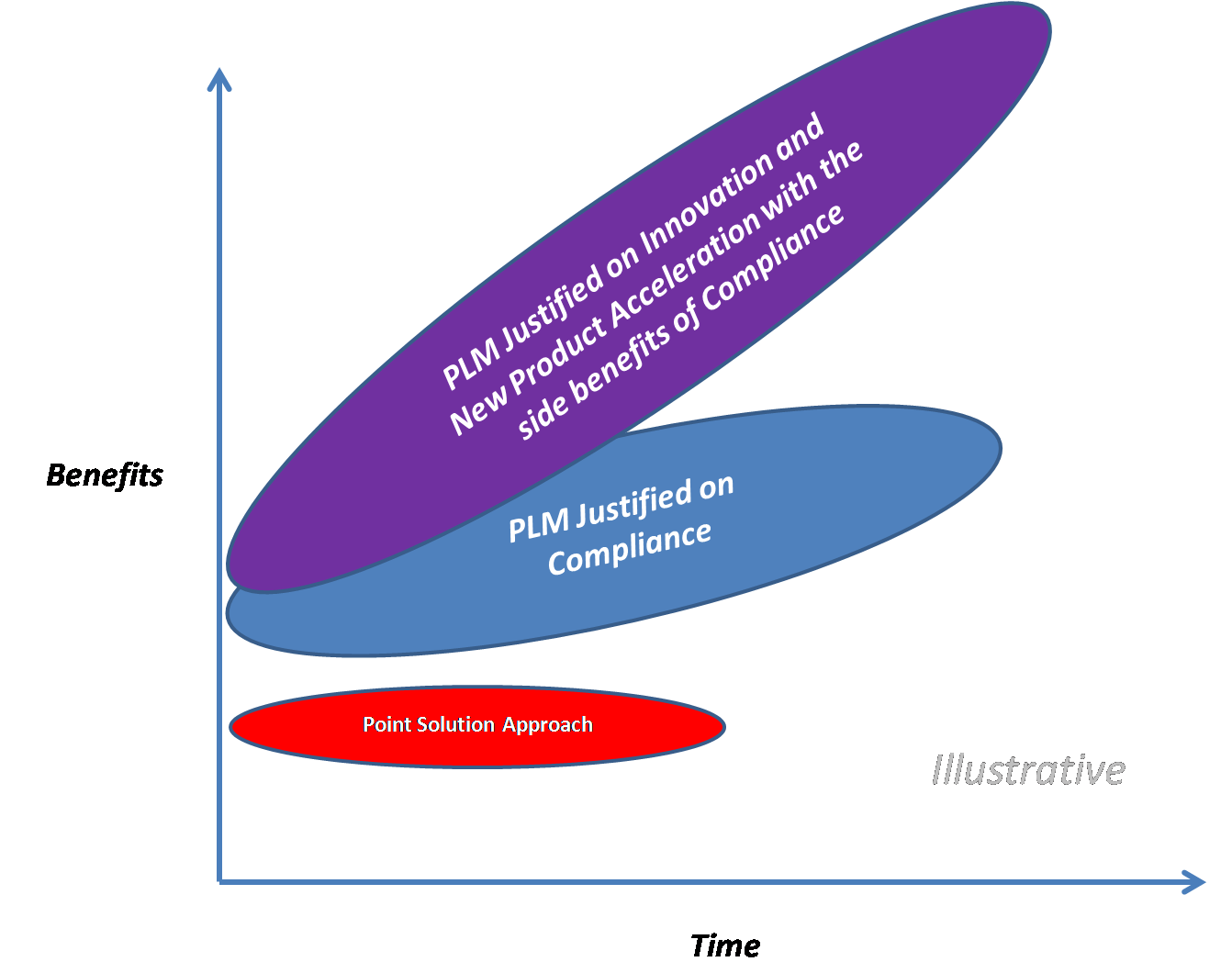
Justifying PLM: A New Perspective on the Investment for Life Sciences Companies
I have been calling directly into the Life Sciences (LS) space for about a year and a half. I will not call myself an expert, but given that I have been in the business world for over 30 years, I feel I have crossed over into the category of a “gray hair” and therefore I can have some relevant observations. My below observations are in some ways provided as an outsider looking into the LS industry.
Tags: Integware, Life Sciences PLM, Regulatory Compliance, PLM Investment, Life Sciences, product lifecycle management, Total product lifecycle managment, Quality Management System, QMS
What do PLM and Tim Tebow have in common?
Tags: NCR, PLM, Integware, Quality system management, Life Sciences PLM, Regulatory Compliance, BOM Management, PLM Insider, Complaint Management, CAPA, Quality Documentation, Life Sciences, PLM implementation, Change Control, product lifecycle management, Total product lifecycle managment, PLM system, PLM deployment, Document Management, PLMinsider, Audit Management, FDA Audits