Product Definition Knowledge Management (PDKM) is a solution architecture approach that guides the use of PLM in supporting the full lifecycle of a product. The purpose is to have all the relevant product information in one place, connected to meet business needs, and safe. In other words, PDKM is a single source of the truth. The PDKM solution covers the product definition scientific and engineering information from the earliest research through all the improvements made to the product and its manufacturing process until the end-of-life.
The business goal of the PDKM solution is to support information reuse across multiple products, just like standard parts promote reuse. “File, Find it, Reuse it”, is the mantra behind the PDKM approach. Why re-do a clinical study if an existing one provides the correct evidence for design verification or design validation. The time and effort of the R&D people is not wasted on re-doing work, but directed towards new product development. This is an operational efficiency improvement for R&D and a product quality improvement because previously approved information is reused.
Within the PDKM solution there are 3 categories of information:
- Product specific information, which is unique to each product that will be approved for sale,
- Sharable approved information that can withstand a regulatory audit and can be referenced by multiple products as evidence,
- Early research information that might or might not lead to a product.
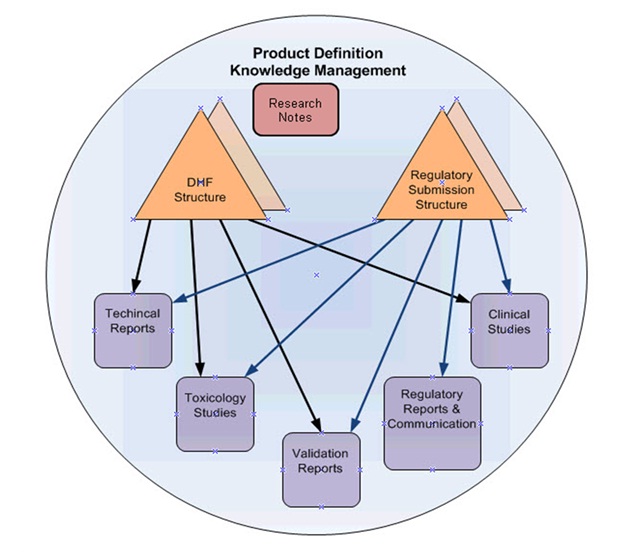
The PDKM approach uses the structuring and referencing capabilities of the PLM platform to organize the scientific and engineering product specific information. For example, structures are used for:
- The DHF, where information required by 21 CFR 820.30 is placed into a product specific structure.
- Within the DHF there is a separate sub-structure to support the traceability from Design Inputs to Design Outputs to Design Verifications and Design Validations.
- Regulatory submissions whether a 510(k), a PMA, or a NDA are structures. One for each product.
Sharable information is organized into Libraries just as shared parts are often organized into libraries, i.e. bolts, brackets, screws, etc. In the PDKM approach the Libraries represent information subject matter areas, such as Toxicology Studies, Clinical Studies, Test Methods, etc. Each Library has its own quality criteria, as long as the information can withstand a regulatory audit and is under the control of the appropriate subject matter experts. Where needed quality and regulatory people can be involved in review and approvals.
References from the product specific structures will be made to items in the Library. For example, a reference from a Design Validation item in the DHF is made to a Clinical Study that is the evidence that the corresponding Design Input is satisfied. The corresponding regulatory submission will make a reference to the same Clinical Study.
Early research information is also kept in subject matter Libraries. The main difference to sharable information is that the research information is less well defined. We want the researchers to record their findings, but because we are dealing with researchers the process needs to be lighter weight. This information is never shown to a regulatory auditor. A failure as much as a success is valuable information. It is enough in most cases to have the author “lock” the information to prevent corruption, although in some cases a peer review is appropriate.
All approvals with the PDKM solution are compliant with Part 11 electronic signatures.
A key business decision is identifying what information is potentially sharable and describing that information in a form that allows for reuse. All information items in the PDKM solution have appropriate meta-data properties to facilitate easy search and retrieval.
In summary the PDKM is intended to provide the re-use benefits to the non-part information needed to define a life sciences product. File it, Find it, Reuse it rather than redo the work.
Dan Matheson
Senior Architect, Integware
