These comments are inspired by two things: 1) observations during PLM consulting work in life science companies and 2) remembering an article by Edeger W. Dijkstra, A Case Against the GO TO Statement, in Communications of the ACM 11 (1968), 3: 147–148. As an R&D product development engineer, I have found the following a great help to innovation: change the vocabulary to change the perspective on the problem, the new perspective enables new insights for innovation.
Dan Matheson
Recent Posts
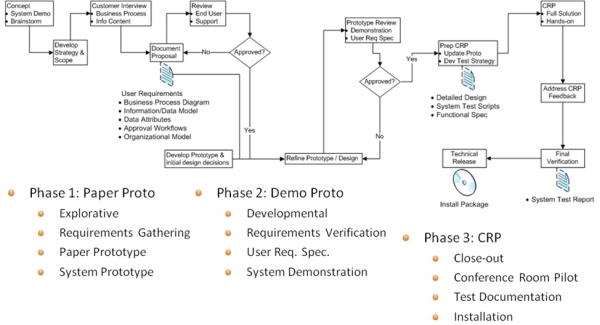
This posting describes an approach to PLM implementations when an OOTB approach is not feasible and following this process leads to the characteristics a first rate PLM implementation. The engagement should be run in an agile manner with frequent deliverables that can be signed-off. The deliverables start as light-weight easily refined components of a Paper Prototype (PP) and evolve with feedback to a Demo Prototype (DP) and finally to a Conference Room Pilot Prototype (CRP) that shows the total solution. Each prototype is signed off by the business users. The purpose of the prototype refinement is to gradually transition the user from a paper-based world into an understanding of a PLM-based solution.
Tags: PLM implementation engagement process, demo prototype, CRP, paper-based, plm platform, PLM, Regulatory Submission, plm solution, PLM implementation, PLM system, DHF, clinical studies, Paper prototype, Conference room prototype, ISO 10746-1
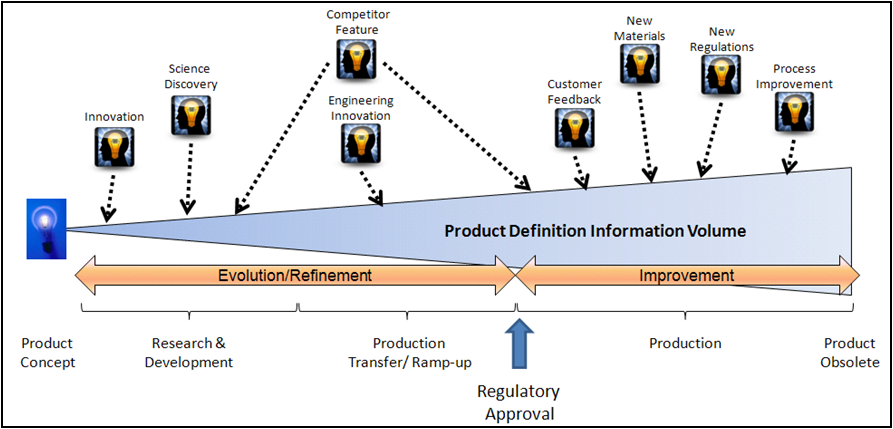
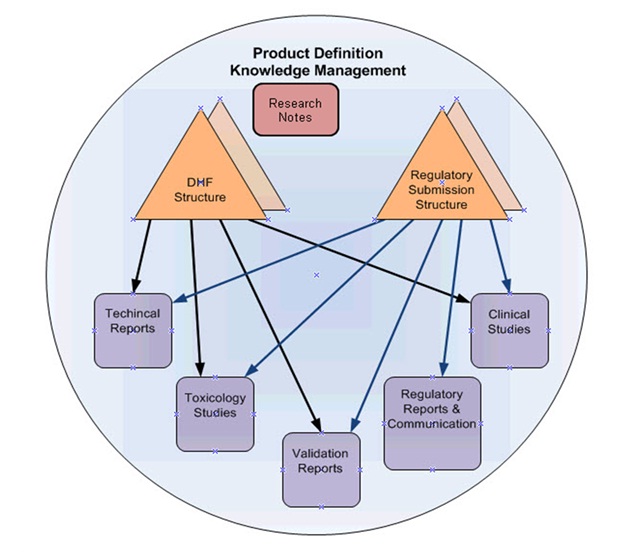
Product Definition Knowledge Management (PDKM) is a solution architecture approach that guides the use of PLM in supporting the full lifecycle of a product. The purpose is to have all the relevant product information in one place, connected to meet business needs, and safe. In other words, PDKM is a single source of the truth. The PDKM solution covers the product definition scientific and engineering information from the earliest research through all the improvements made to the product and its manufacturing process until the end-of-life.
Tags: 510(k), PDKM, NDA, PLM, Product Development, design history file, Product Definition Knowlege Management, plm solution, PLM system, DHF, clinical studies, Product lifecycle, 21 CFR 820.30