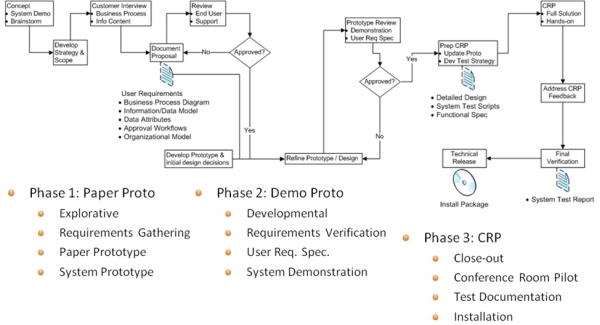
This posting describes an approach to PLM implementations when an OOTB approach is not feasible and following this process leads to the characteristics a first rate PLM implementation. The engagement should be run in an agile manner with frequent deliverables that can be signed-off. The deliverables start as light-weight easily refined components of a Paper Prototype (PP) and evolve with feedback to a Demo Prototype (DP) and finally to a Conference Room Pilot Prototype (CRP) that shows the total solution. Each prototype is signed off by the business users. The purpose of the prototype refinement is to gradually transition the user from a paper-based world into an understanding of a PLM-based solution.
Tags: PLM implementation engagement process, demo prototype, CRP, paper-based, plm platform, PLM, Regulatory Submission, plm solution, PLM implementation, PLM system, DHF, clinical studies, Paper prototype, Conference room prototype, ISO 10746-1
Now that spring is here and summer is on the horizon and if you are like me you want to look your best for summer. What does that mean? Usually a diet of some sort, but what do you look for when starting a diet and how am I going to apply that to PLM?
Tags: PLM, Integware, Complaint Management, PLM implementation, PLM system, PLM deployment, iC5 Turbo
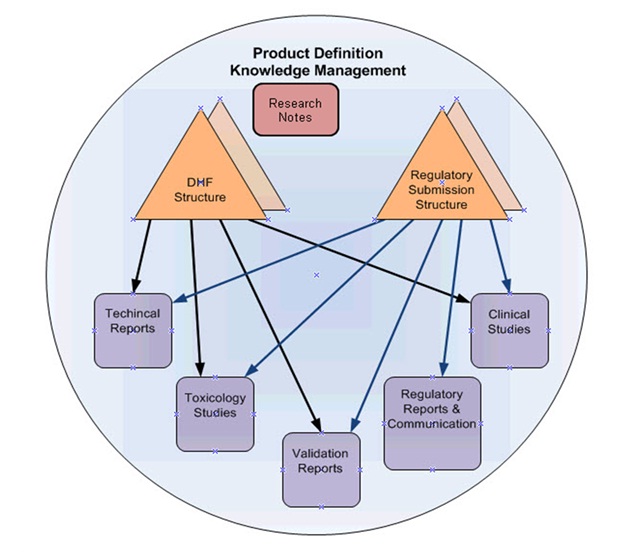
Product Definition Knowledge Management (PDKM) is a solution architecture approach that guides the use of PLM in supporting the full lifecycle of a product. The purpose is to have all the relevant product information in one place, connected to meet business needs, and safe. In other words, PDKM is a single source of the truth. The PDKM solution covers the product definition scientific and engineering information from the earliest research through all the improvements made to the product and its manufacturing process until the end-of-life.
Tags: 510(k), PDKM, NDA, PLM, Product Development, design history file, Product Definition Knowlege Management, plm solution, PLM system, DHF, clinical studies, Product lifecycle, 21 CFR 820.30
Build a better BOM using PLM and what not to say in an airport
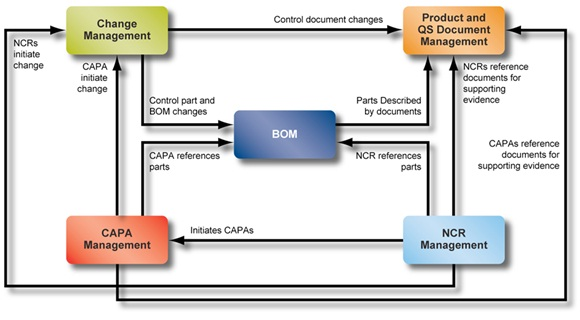
During a recent business trip, I was sitting in an airport café with some colleagues while discussing how to build and manage BOMs. We got some strange looks. Then I mentioned something about exploding the BOM. More strange looks. Nervous glances. Finally, we decided it was best to avoid using the B word in airports. But since you probably know a thing or two about BOMs and PLM (otherwise, uh, why are you reading this?), I'll assume you know what I'm talking about and I can use my normal Nerd-speak of PLM acronyms. (My nickname around the office is "Big Nerd", because I'm six foot four and I think BOMs are interesting).
Tags: NCR, PLM, BOM Management, design history file, Change management, CAPA, DMR Management, PLM system, BOM, DHF, Document Management, DMR
What do PLM and Tim Tebow have in common?
Tags: NCR, PLM, Integware, Quality system management, Life Sciences PLM, Regulatory Compliance, BOM Management, PLM Insider, Complaint Management, CAPA, Quality Documentation, Life Sciences, PLM implementation, Change Control, product lifecycle management, Total product lifecycle managment, PLM system, PLM deployment, Document Management, PLMinsider, Audit Management, FDA Audits